Snakebites have challenged humans for centuries. Without a proper understanding of how snake venoms worked, physicians used the same ineffective treatments through much of history. These practices included manually sucking the venom out of a wound or serving Theriac, a concoction made of herbs, spices, opium, ground-up snakes, and even powdered mummies, to the victim. These remedies remained popular in western medicine into the seventeenth century.1
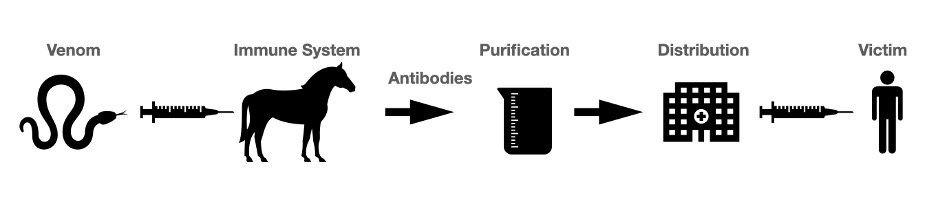
Antivenom, or antivenin, was developed in the 1890s as a new treatment for envenomation. Though who first created antivenom is heavily debated, most credit a French immunologist named Albert Calmette (1863-1933) who sought a treatment for cobra envenomations in Vietnam. Having witnessed a large number of monocle cobra bites after a heavy rainy season, he decided he had to act. He developed a new serum by injecting small, non-lethal doses of cobra venom into various animals; these animals went on to develop antibodies capable of neutralizing the venom. Calmette would then extract some blood from the animals and isolate the antibodies to purify into antivenom.2 This technique is still used in animals such as horses and goats to create antivenom today.3
Calmette’s discovery eventually led to commercial production of antivenoms. Three decades later, the HK Mulford Company of Philadelphia, overseen by the Antivenin Institute of America, began producing the first commercial antivenom in the United States. Their antivenom, called the Antivenin Nearctic Crotalidae, could treat the bites of various North American crotalids (copperheads, cottonmouths, and rattlesnakes). This versatility was possible because of the antivenom’s polyvalence–it contained antibodies effective against the venoms of numerous species.4
Antivenoms are now manufactured all over the world to treat all kinds of snakebites. However, the technology is far from perfect. Polyvalent antivenoms continue to be a work in progress today, and one of the main difficulties in creating a versatile and effective antivenom stems from the variation in venom across species. Elapids like cobras and taipans, for example, usually possess neurotoxic venom, while crotalids such as rattlesnakes and adders possess a completely different hemotoxic venom.
Due to significant diversity in venoms, drug companies must develop antivenoms specific to each snake species. Existing polyvalent antivenoms are limited and can only treat a few of the species most responsible for envenomations. For example, the main polyvalent antivenom produced in India only covers the “Big 4” snake species out of the 60 capable of envenoming humans. As a result, there is no antivenom treatment for people who have been bitten by many snakes not included in the “Big 4.” Without any other available treatment, doctors are forced to use this same antivenom on these patients, often leading to treatment failure. Furthermore, all Indian antivenom manufacturers source their venoms from one geographical population of each species, and because of variations within different populations of the same species, these antivenoms may not be very effective for patients of different locations.5 Similar issues exist with antivenoms produced around the world.
Current antivenom technology is limited due to the complexities of venom across and within species. However, scientists are creating new potential therapies with the goal of more effectively treating all snakebites. For example, the drug manufacturer Ophirex is conducting clinical trials for a PLA2 inhibitor, varespladib, which could treat snakebites from more species than any current treatment. Unlike traditional antivenom technology, the drug works by blocking PLA2 enzymes that play a major role in bleeding, tissue destruction, and paralysis. This class of enzymes are found in 95% of all snake venoms and may be the key to developing treatments that cover snakebites more broadly. Other small molecule therapeutics such as metalloproteinase inhibitors, aptamers, and chelators, among many others, are also currently being studied. As companies experiment with new therapies, antivenom technology will continue to grow and improve, hopefully resulting in better health outcomes for snakebite patients around the world.6
If you found this article informative, please consider donating to ASF today. Every donation is 100% tax deductible and goes directly to patient care in Africa.
Casewell, N. R., Jackson, T., Laustsen, A. H., & Sunagar, K. (2020). Causes and Consequences of Snake Venom Variation. Trends in pharmacological sciences, 41(8), 570–581. https://doi.org/10.1016/j.tips.2020.05.006